Polymers are made up of various molecules which combine together to form long chains. They usually have high melting and boiling points like PVC (poly vinyl chloride), polystyrene, cellulose. Therefore, the list of polymers with their uses is helpful in the academic and competitive exam preparations.
Polymers are made up of various molecules which combine together to form long chains. Polymers usually have high melting and boiling points like PVC (poly vinyl chloride), polystyrene, cellulose. These simple molecules which bind to form polymers are known as monomers.
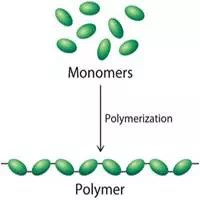
Monomers are the building blocks of more complex molecules, called polymers. Therefore, we can say that a monomer is a molecule that forms the basic unit for polymers and they bind with other monomers to form a repeated chain molecule. Like glucose, vinyl chloride, amino acids etc.
Characteristics of polymers are:
a) They are made, by addition or by condensation.
b) They are homopolymers or heteropolymers (co-polymers).
c) They are themoplastics, thermosets, elastomers or fibres.
d) Have steric structure.
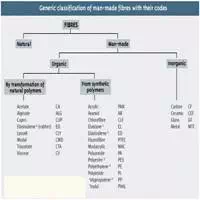
List of important Synthetic fibres
List of Some common man-made Polymers and their Uses:
| S. No. | Polymer | Use |
| 1. | Polythene | Packaging, material, carry bags, bottles. |
| 2. | Teflon | Nonstick Kitchen ware |
| 3. | Polypropene | Bottles, Crates |
| 4. | Melamine | Crockery |
| 5. | Polyvinyl chloride (PVC) | Pipes Insulation |
| 6. | Lexan | Bullet proof glass |
| 7. | Vinyl rubber | Rubber erasers |
| 8. | Bakelite | Electrical insulation buttons |
| 9. | Polystyrene | Foam Thermocole |
| 10. | Poly (Styrene butadiene) | Rubber bubble gum |
| 11. | Nylon (Polyester) | Fibres, ropes |
| 12. | Luminous Paints | Glow when exposed to light. They are applied on a surface to protect it from corrosion and weathering. |
| 13. | Antimicrobial polymers (polymeric biocide) | The ability to inhibit the growth of microorganisms |
| 14. | Antigen | Substance capable of stimulating formation of antibodies. |
| 15. | Antipyretie | A substance used to lower body temperature. |
| 16. | Pesticides | Used to kill animals |
| 17. | Para-aramid fibre (Kevlar) | Manufacturing armour, sports and musical equipment. Used in the field of cryogenics. |
| 18. | Polyacrylonitrile (PAN) (Orlon) | Used for making clothes and fabrics like sweaters, hats,rugs, etc |
| 19. | Copolyamid (Technora) | Used for manufacturing optical fiber cables, drumheads, automotive industry, ropes, wire ropes and cables. |
| 20. | Polytetrafluoroethylene (PTFE)(Viton) | Viton B is used in chemical process plants and gaskets. As it depends upon the grade of the polymer. |
We have seen the list of polymers with their uses. But one question arises in my mind that how polymers are formed.
They are formed with the help of a process known as polymerization. It is the process of binding smaller monomers into the polymers by covalent bond. During polymerization, chemical groups are lost from the monomers so that they may join together. In the case of biopolymers of carbohydrates, this is a dehydration reaction in which water is formed.