As will be noted shortly, certain techniques such as colligative methods (Secs. 3.6–3.8), light scattering photometry, special mass spectral techniques, and ultracentrifugation allow the calculation of specific or absolute molecular weights. Under certain conditions some of these allow allow the calculation of the molecular weight distribution (MWD). These are a wide variety of chromatography techniques including paper and column techniques. Chromatographic techniques involve passing a solution containing the to-betested sample through a medium that shows selective absorption for the different components in the solution. Ion exchange chromatography separates molecules on the basis of their electrical charge. Ion exchange resins are either polyanions or polycations. For a polycation resin, those particles that are least attracted to the resin will flow more rapidly through the column and be emitted from the column first.
This technique is most useful for polymers that contain changed moieties. In affinity chromatography, the resin contains molecules that are especially selected that will interact with the particular polymer(s) under study. Thus, for a particular protein, the resin may be modified to contain a molecule that interacts with that protein type. The solution containing the mixture is passed through the column and the modified resin preferentially associates with the desired protein allowing it to be preferentially removed from the solution. Later, the protein is washed through the column by addition of a salt solution and collected for further evaluation. In high-performance liquid chromatography (HPLC), pressure is applied to the column that causes the solution to rapidly pass through the column allowing procedures to be completed in a fraction of the time in comparison to regular chromatography.
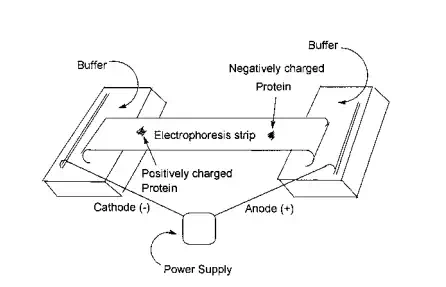
When an electric field is applied to a solution, polymers containing a charge will move toward either the cathode (positively charged species) or the anode (negatively charged species). This migration is called electrophoresis. The velocity at which molecules move is mainly dependent on the electric field and change on the polymer driving the molecule toward one of the electrodes, and a frictional force dependent on the size and structure of the macromolecules that opposes the movement. In general, the larger and more bulky the macromolecule, the greater the resistance to movement, and the greater the applied field and charge on the molecule the more rapid the movement. While electrophoresis can be conducted on solutions it is customary to use a supporting medium of a paper or gel. For a given system, it is possible to calibrate the rate of flow with the molecular weight and/or size of the molecule. Here the flow characteristics of the calibration material must be similar to those of the unknown.
Generally though, electrophoresis is often employed in the separation of complex molecules such as proteins where the primary factor in the separation is the charge on the species. Some amino acids such as aspartic acid and glutamic acid contain an “additional” acid functional group, while amino acids such as lysine, arginine, and histidine contain “additional” basic groups. The presence of these units will confer to the protein tendencies to move towards the anode or cathode. The rate of movement is dependent on a number of factors including the relative abundance and accessability of these acid and base functional groups. Figure 3.8 contains an illustration of the basic components of a typical electrophoresis apparatus. The troughs at either and contain an electrolyte buffer solution. The sample to be separated is placed in the approximate center of the electrophoresis strip. Gel permeation chromatography (GPC) is a form of chromatography that is based on separation by molecular size rather than chemical properties. GPC or size exclusion
chromatography (SEC) is widely used for molecular weight and MWD determination. In itself, SEC does not give an absolute molecular weight and must be calibrated against polymer samples whose molecular weight has been determined by a technique that does give an absolute molecular weight. Size exclusion chromatography is an HPLC technique whereby the polymer chains are separated according to differences in hydrodynamic volume.
This separation is made possible by use of special packing material in the column. The packing material is usually polymeric porous spheres, often composed of polystyrene crosslinked by addition of varying amounts of divinylbenzene. Retension in the column is mainly governed by the partitioning (or exchanging) of polymer chains between the mobile (or eluent) phase flowing through the column and the stagnate liquid phase that is present in the interior of the packing material. Through control of the amount of crosslinking, nature of the packing material and specific processing procedures, spheres of widely varying porosity are available.
The motion in and out of the stationary phase is dependent on a number of factors including Brownian motion, chain size, and conformation. The latter two are related to the polymer chain’s hydrodynamic volume—the real, excluded volume occupied by the polymer chain. Since smaller chains preferentially permeate the gel particles, the largest chains are eluted first. As noted above, the fractions are separated on the basis of size. The resulting chromatogram is then a molecular size distribution (MSD).
The relationship between molecular size and molecular weight is dependent on the conformation of the polymer in solution. As long as the polymer conformation remains constant, which is generally the case, molecular size increases with increase in molecular weight. The precise relationship between molecular size and molecular weight is conformation-dependent. For random coils, molecular size as measured by the polymer’s radius of gyration, R, and molecular weight, M, is proportional to Mb , where b is a constant dependent on the solvent, polymer concentration, and temperature. Such values are known and appear in the literature for many polymers, allowing the ready conversion of molecular size data collected by SEC into molecular weight and MWD.
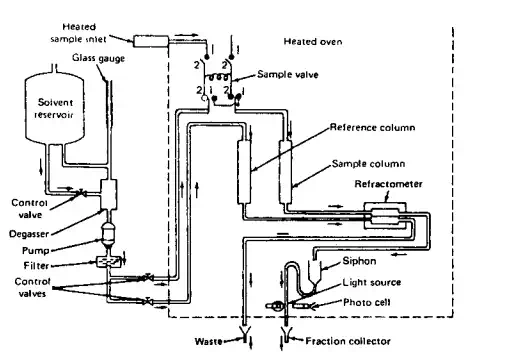
There is a wide variety of instrumentation ranging from simple manually operated devices to completely automated systems. Figure 3.9 contains a brief sketch of one system. Briefly, the polymer-containing solution and solvent alone are introduced into the system and pumped through separate columns at a specific rate. The differences in refractive index between the solvent itself and polymer solution are determined using a differential refractometer. This allows calculation of the amount of polymer present as the solution passes out of the column
