Average Molecular Mass
Increasing the average molecular mass of a polymer simply means increasing the average length of the molecular chains that make up its structure.
The strength of the polymer increases with the molecular mass. However, employing molecular mass as a means of improving strength is rare in industry because, beyond a certain level, further increases in molecular mass have little effect on polymer strength.
The most notable example of this in industry is the difference in mechanical properties found between low-density polyethylene, LDPE, and ultra high molecular weight polyethylene, UHMWPE. The extremely high molecular weight found in UHMWPE provides this polymer with exceptionally high toughness and wear resistance.
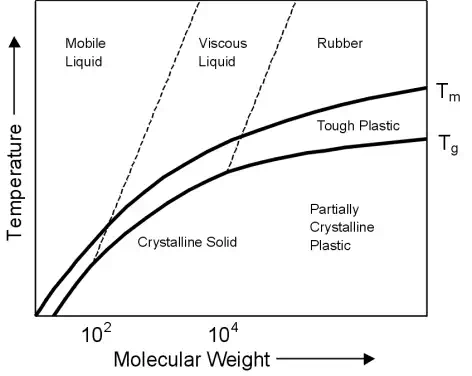
The effect of molecular weight on the melting temperature and glass transition temperature can be seen in the graph shown. An increase in temperature will have the reverse effect to an increase in molecular weight. A higher molecular weight is likely to produce a polymer with a high Tm and Tg and a high toughness.
After Materials Science and Engineering: An Introduction, William D. Callister, 5th Edition, John Wiley & Sons.
Plasticisers
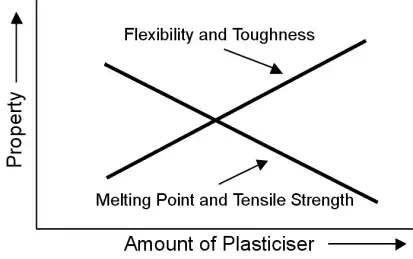
Plasticisers are organic liquids that are added as a means of improving flexibility and toughness. The most commonly encountered plasticised polymer in industry is PVC where the plasticiser is added to increase the polymer’s internal free volume thus providing more space within the polymer for motion of the polymer chains.
Although plasticisers are excellent for increasing the flexibility of a polymer they have adverse side effects in that they reduce both the Tg and tensile strength of the plastic.

