There are 3 principal classes of polymers – thermoplastics, thermosets, and elastomers. Differentiation between these classes is best defined by their behaviour under applied heat.
· Thermoplastic polymers can be either amorphous or crystalline. They behave in a relatively ductile manner but often have low strength.
· Thermosetting polymers are always amorphous and are generally strong and rigid but often brittle.
· Elastomers are always amorphous and are used in service above their Tg. They have the unique ability to elastically deform by extremely large amounts without permanent damage to their shape.
Thermoplastics
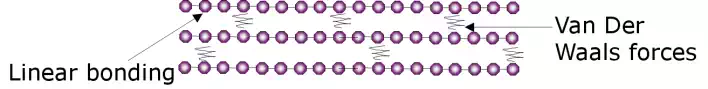
Thermoplastics may take on amorphous or crystalline structures.
In thermoplastics the long chain molecules exist in the form of linear bonding but are also bonded to each other by secondary Van Der Waals forces (secondary bonds).
At a high enough heat the excitation of the molecular chains is enough to overcome this binding force and they are free to move over one another thereby creating a viscous liquid. The secondary bonds can be envisaged to have melted. The glass transition (Tg) temperature can be envisaged as the temperature at which the secondary bonds melt.
When the polymer is cooled the secondary forces once again dominate and the molecular chains revert back to a restricted state. This means that thermoplastics can be melted and remelted allowing them to be easily recycled.
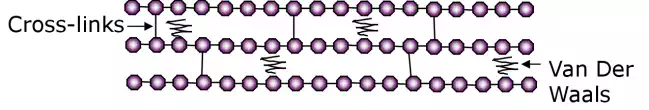
Thermosets
In thermosetting plastics the long chain molecules exist in an amorphous network with cross-linked bonding. This means that the long molecular chains are attached to each other by covalent bonds. The formation of these cross-links is known as curing.
Cross-linking sets the molecular chains in place and therefore means that a thermosetting plastic cannot be remelted but will instead decompose upon being heated to a temperature above the Tg.
Cross-linking inhibits molecular arrangement into an ordered crystalline structure meaning that thermosetting polymers only exist in the amorphous state.
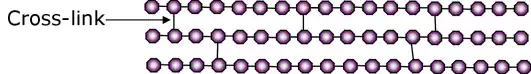
Elastomers
In elastomers the long molecular chains exist in the form of amorphous linear bonding with occasional cross-linking.
At room temperature the level of excitation of the chains has already overcome the secondary Van Der Waals bonds, however, the cross-links that exist in the structure act to revert the elastomer back to its original form following deformation.